This article goes through the resources and information Grainfather uses to calculate IBU’s on the recipe creator.
If you'd like to have a listen to a bit of an overview on this topic, our experts are featured on this podcast explaining more about IBU in relation to hop usage.
International Bitterness Units
IBU or International Bitterness Unit was created to help brewers make beer with a consistent level of bitterness. The IBU is a measure of the Isomerised alpha acids and non-isomerised hop material in a finished beer when analysing by UV-Vis spectroscopy then multiplied by a correction factor to get the expressed overall bitterness in beers from the 1960s (Hieronymus, 2012).
There are, however, several shortcomings of the IBU. Since this method was developed using beers in the 1960s where the hops they were using in the brewing process were very oxidised compared to those we get today, which for the beers of the 1960s worked well. Still, due to cold storage, high alpha hops with low beta acids and the use of hop products less susceptible to oxidation means the factor used in the original calculation may not be a true representative of the bitterness of beers today (Peacock, 2007). Today high-pressure liquid chromatography is used to measure iso-alpha acids, but this is unique to the beer brewed under their specific conditions. Therefore, brewers, today benefit from the IBU, as a tool when formulating recipes and maintaining bitterness levels in regularly brewed beers while recognising that the IBU does not entirely reflect the quality of the bitterness or the overall perception of bitterness. Or many small breweries rely on the estimates as they don’t have access to the laboratories or don’t see the value in testing.
Tinseth, Rager, Garetz, Mosher, Noonan or Daniels all have equations that can be used to calculate IBUs when brewers are developing recipes and the key differences being how each of them approached Hop Utilisation, as seen in the figure below.
Figure 1: Utilisation Rate as a Function of Boil Time and Simple beer IBU (Michael L. Hall, 1997)
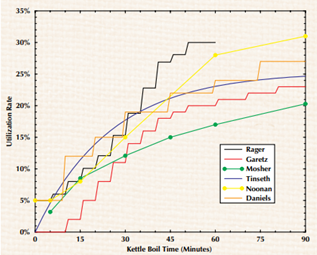
Utilisation rate as a function of boil time for wort of SG 1.050 boiled at sea level using low hopping rate with fresh leaf hops without a hop bag. With an average yeast flocculation and no filtration.
The Grainfather Brewing community tools offers a choice of the following bitterness methods:
- Tinseth
- Rager
- Garetz
- Daniel
- mIBU
Our recommended method (and default going forward in the recipe creator) is the mIBU method, developed by Paul-John Hosom. It is based on the Tinseth equation but includes many optimisations such as a way to model post-boil bitterness contributions by taking into account additional equipment/process parameters. It does this by modelling your post-boil temperature throughout hop stand (if applicable) and cooling stages, and then calculates the corresponding IBU contributions based on those temperatures. It also includes an option to apply alpha-acid solubility limit correction.
For more information about the mIBU technique see the full article here.
The Glen Tinseth equation for predicting IBU is the most used calculation for calculating hop utilisation during the boil. Since Tinseths research was compiled, there has been some modifications and improvements have been made when calculating flameout, whirlpool and low-temperature hop additions. However, with any calculation, many variables affect Utilisation. (Hieronymus, 2012)
- Hop form – whole, fresh, pallets, extracts etc. Hop pallets are ~10-15% more efficient than hop cones.
- The limited solubility of Alpha acid and Iso alpha acid in wort.
- Boiling time and vigour
- The relationship between time and Utilisation is non-linear
- Boils longer than 90 mins iso-alpha acids start to break down to unidentified and undesirable components.
- Kettle Geometry larger kettles are more efficient. Approximately 20% more efficient between a 5 gal (23L) batch and a small commercial batch 132 gals (500L)
- Wort Gravity, utilisation decreases as gravity increases. However, as the alcohol and unfermented carbohydrates increase the IBU potential increases.
- pH and its effect on the production of auxiliary bittering compounds and AA solubility limits
Figure 2 Existing methods of IBU vs Gravity (Hosom, 2018)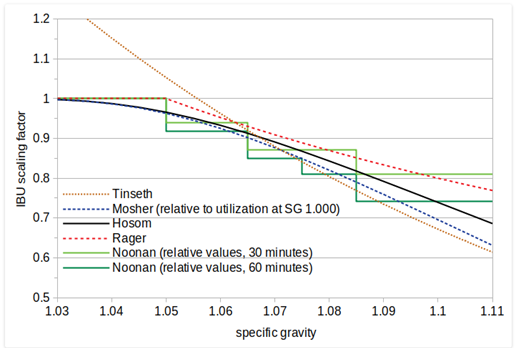
A visual comparison of existing methods (and the formula developed here, labelled ‘Hosom’) for accounting for the effect of specific gravity on IBUs.
- Boil temperature, altitude affects boil temperature and boiling at lower temps (higher altitudes) results in lower Utilisation.
- The composition of Humulones. Hops with higher cohumulone are more efficient.
Table 1 Tinseth IBU equation (Tinseth, 1997)
|
Definition |
Formula |
Symbol |
|
Hop Utilisation |
= b(BG) x f(t1) |
U |
|
Bigness Factor |
= 1.65 x 0.000125^(OG + predicted preboil G)/2) |
b(BG) |
|
Original gravity |
- |
OG |
|
Boil Time Function |
= |
f(t1) |
|
Boil time (mins) |
- |
t |
|
Density of AA in Wort mg/L |
D |
|
|
Alpha Acid |
- |
AA |
|
Weight of hops in grams |
- |
w |
|
Final Kettle volume |
- |
V |
|
IBU Calculation |
U * D |
IBU |
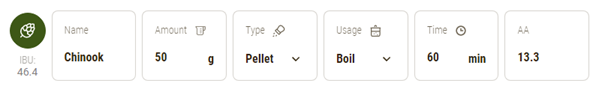
Example: Boil Hops
|
Calculator |
Hop |
AA |
Addition time remaining |
t |
V |
OG |
|
BC |
Chinook |
13.3 |
60 |
60 |
30 |
1.061 |
|
Symbol |
Brewing Community |
|
b(BG) |
0.954 |
|
f(t1) |
0.2191 |
|
D |
221.67 |
|
U |
0.214 |
|
IBU |
46.4 |
Flameout/ Whirlpool Hop IBU
With Tinseth, Rager, Garetz, Mosher, Noonan or Daniels, the predicted IBU for post-boil hops is zero. However, even at temperatures of down to 790C (1750F), there is still some alpha acid isomerisation. (Raspuzzi, n.d.).
For calculating hop bitterness in the whirlpool is similar to calculating it in the boil. Hop bitterness is a function of boil (or steep) time, amount of hops used, boil volume, boil gravity, and the alpha acid percentage of the hops. The same fundamental relationships apply to the whirlpool. The only significant difference is that in the whirlpool, the hops are at a lower temperature, so the isomerisation process takes place at a much slower rate. Below boiling the isomerisation rate drops off very rapidly. As a result the temperature and time of your whirlpool additions are vital. if we calculate the hop utilisation for an equivalent boil hop using the time, volume and gravity of the wort for the addition. In that case, we can then apply the “whirlpool utilisation” to this number to estimate the overall hop utilisation.
Our approach using the Tinseth/Rager/Garetz/Daniel equations for predicting IBUs for post-boil hops is as follows:
T = Temperature in Kelvin
|
Temperature |
Utilisation Factor (U) |
|
90°C (194 F) |
49% |
|
80°C (176 F) |
23% |
|
70°C (158 F) |
10% |
|
60°C (140 F) |
4.3% |
|
50°C (122 F) |
1.75% |
The main reason we calculate whirlpool hop utilisation is so we don’t accidentally upset the IBU balance of our beer when using a large amount of whirlpool hops.
However, A consequence of boil additions often being overlooked is the factor that the effect of boil hops that carry into the whirlpool. For example, for a 10-minute addition, the hops will generate some bitterness in the boil, but it will still have quite a bit of alpha acid left as we move into the whirlpool. So its remaining alpha acids will continue to generate some bitterness in the whirlpool. For this calculation we recommend using the mIBU calculation.
For mIBU:
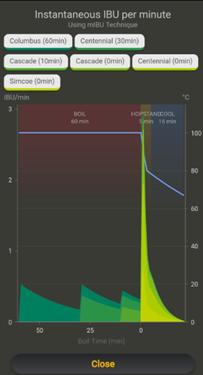
The mIBU method includes comprehensive models for post-boil IBU contributions for all hop additions across both the boil, hopstand and cooling/transfer phases. It considers extra factors like equipment kettle diameter, cooling method and cooling parameters, hopstand process, and hop temperature to predict the IBU contribution over time. Use our mIBU chart to visualise how all these factors affect your bitterness.
References
Hieronymus, S. (2012). For The Love of Hops. Boulder, Colorado, USA: Kristi Switzer.
Hosom, J.-P. (2018, 5 7). Specific Gravity and IBUs. Retrieved from https://alchemyoverlord.wordpress.com/: https://alchemyoverlord.wordpress.com/#references_SG
Michael L. Hall, P. (1997). Whats Your IBU. Retrieved from Homebrewers Association: https://www.homebrewersassociation.org/attachments/0000/2501/IBUs.pdf
Peacock, V. (2007). Hop Chemistry 201. Craft Brewers Conference. Austin, Texas.
Raspuzzi, D. (n.d.). Hop Stands-2. Retrieved from Brew Your Own: https://byo.com/article/hop-stands-2/
Tinseth, G. (1997). Glenn's Hop Utilization Numbers. Retrieved from realbeer.com: https://realbeer.com/hops/research.html